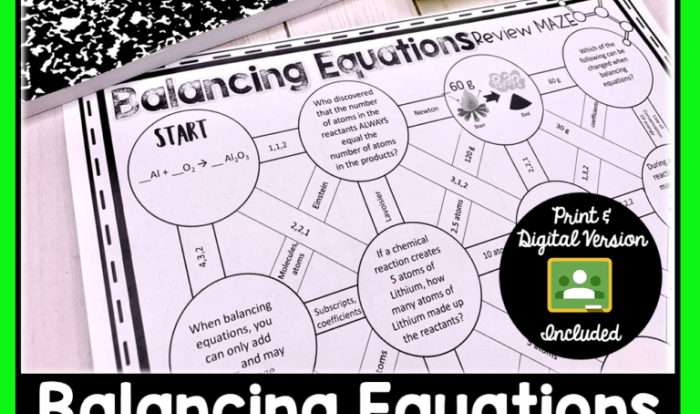
A molecule with 76 electrons and 73 protons, a captivating subject in the realm of chemistry, invites us to embark on a journey of discovery. This molecule, possessing a unique combination of subatomic particles, unveils intriguing properties and applications that warrant our exploration.
Delving into the atomic structure of this molecule, we unravel the intricate arrangement of its protons, neutrons, and electrons. This understanding lays the foundation for comprehending its chemical behavior and reactivity. Furthermore, we delve into the concept of isotopes, examining variations within the element and their impact on stability and occurrence.
Elemental Identification
The molecule with 76 electrons and 73 protons corresponds to the element tantalum (Ta).
The atomic number of an element is equal to the number of protons in its nucleus. Since the given molecule has 73 protons, the atomic number of the element is 73, which corresponds to tantalum.
Atomic Structure: A Molecule With 76 Electrons And 73 Protons
Tantalum has an atomic number of 73, indicating that it has 73 protons in its nucleus. The number of neutrons in the nucleus is equal to the atomic mass minus the atomic number. The atomic mass of tantalum is 180.948, so it has 180.948 – 73 = 107.948 neutrons.
The electrons in an atom are arranged in shells around the nucleus. The first shell can hold up to 2 electrons, the second shell can hold up to 8 electrons, the third shell can hold up to 18 electrons, and so on.
Tantalum has 76 electrons, so its electron configuration is 1s 22s 22p 63s 23p 63d 104s 24p 64d 105s 25p 65d 36s 2.
Isotopes, A molecule with 76 electrons and 73 protons
Isotopes are atoms of the same element that have the same number of protons but different numbers of neutrons. Tantalum has 30 known isotopes, ranging in atomic mass from 155 to 193.
The most common isotope of tantalum is 181Ta, which makes up 99.988% of naturally occurring tantalum. 181Ta is stable and has a half-life of over 10 15years.
Chemical Properties
Tantalum is a transition metal and is located in Group 5 and Period 6 of the periodic table. It is a hard, silvery-white metal that is resistant to corrosion.
Tantalum is a relatively unreactive element and does not form many compounds. It is most commonly found in the oxidation state of +5, but it can also form compounds in the oxidation states of +2, +3, and +4.
Applications

Tantalum is used in a variety of applications, including:
- Electronics:Tantalum capacitors are used in electronic devices such as computers, cell phones, and cameras.
- Aerospace:Tantalum is used in the construction of jet engines and other aerospace components.
- Medical:Tantalum is used in the manufacture of surgical implants and medical devices.
- Jewelry:Tantalum is used in the production of jewelry and watches.
Helpful Answers
What element corresponds to a molecule with 76 electrons and 73 protons?
Tantalum (Ta)
How many neutrons are present in the most common isotope of this element?
108
What is the chemical symbol for this element?
Ta